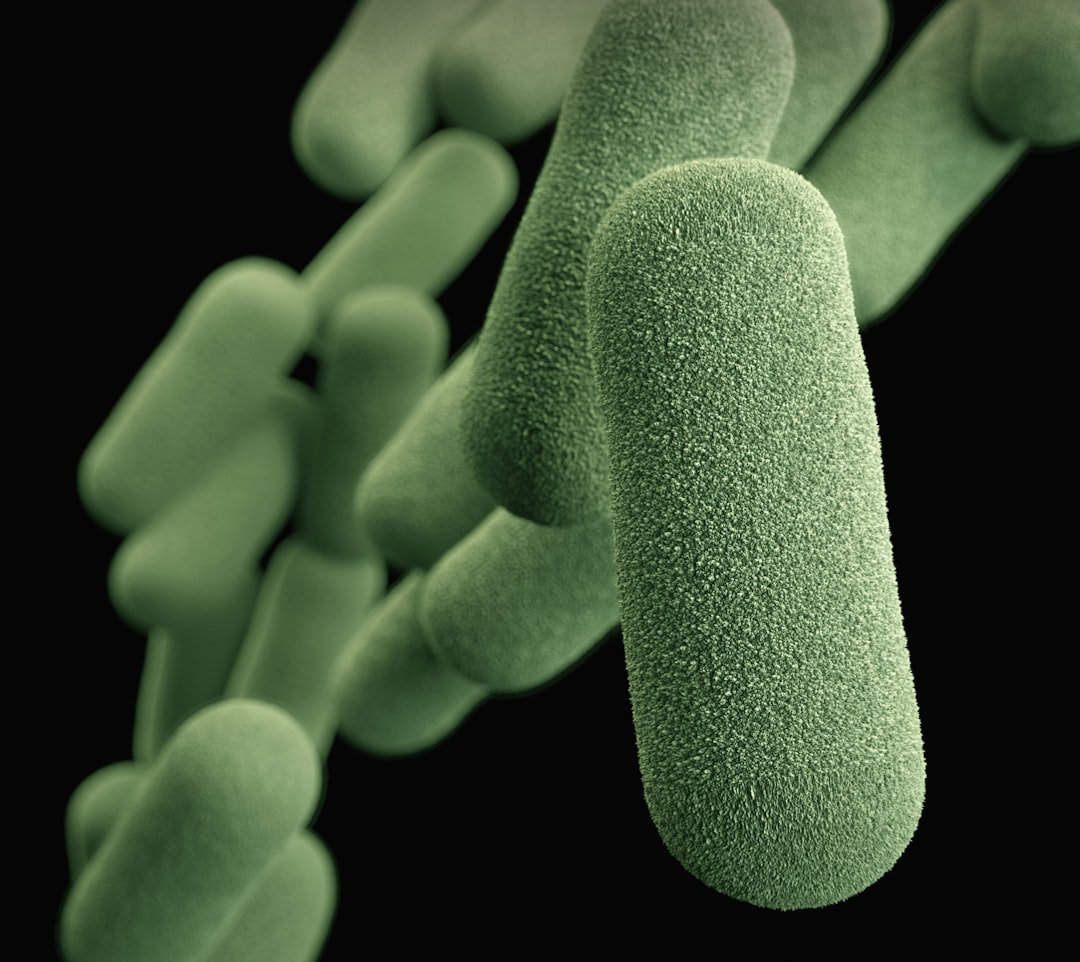
What is it about?
How did antibiotic resistance genes which naturally occur in soil bacteria find their way into other bacteria that live in the gut and can cause antibiotic resistant infections in humans? We wanted to determine how widespread similar bacteria are in the guts of animals in nature, and whether those might be either the source or a conduit for passage of resistance to human strains. Hypothetically, if similar types of Enterococcus bacteria live in the guts of insects and other things that live in the soil where antibiotics have been produced naturally for hundreds of millions of years, some of those enterococci might be naturally antibiotic resistant. When a farm animal that is fed antibiotics, such as a chicken, scratches the ground and eats these insects along with their enterococci, they may then be an important point of collection and amplification of resistant strains that are now connected to the human ecology.
Featured Image

Photo by Bailey Zindel on Unsplash
Why is it important?
Since their discovery almost 100 years ago, humans have produced millions of tons of antibiotics, compounds that naturally exist in the soil but are not naturally present in large quantities in humans, and all of these antibiotics have been applied to humans and to animals and plants associated with humans. This has then led to the movement of antibiotic resistance genes from the soil where they have occurred for hundreds of millions of years, into microbes that inhabit and infect humans. Most of the antibiotics produced are applied in agriculture -- a human activity closely associated with the soil ecology. It remains controversial how important this practice is to the spread of antibiotics in the microbes that infect humans. In this work we found that relatives of microbes that naturally live in the human gut known as enterococci, are very widespread in the guts of animals that live on land. We further found that we have only the most superficial knowledge of those enterococci. In examining about 800 samples of animal feces, insects and other sources from around the world, we found 18 new species of enterococci never before seen. Within these are thousands of new genes that are important to the relationship between the microbe and where they were found. Most of these new species were found in insects and invertebrates, or animals like chickens and dragonflies that eat insects and invertebrates. From the rate of discovery of new enterococci, compared to the number of animal species yet to be explored, we project that there are thousands of species of enterococci yet to be discovered, and many thousands of new genes associated with their lifestyle in nature. We conclude that 90 - 99% or more of enterococcal species and their genes are yet to be discovered. In the comparatively few natural enterococci we have studied, we already have found new types of toxins in addition to the many previously unknown genes that are important for the enterococci to successfully live with their various hosts. Because of this work, we now know where to look to discover new enterococci. Because they live in such diverse land animals, by finding patterns of similarity between species occurring for example in insects versus mammals, we can identify genes of difference that are important for determining their ability to successfully colonize a particular type of animal. With that knowledge, we believe we would be able to develop a strategy for preventing them from colonize an animal where that is not wanted, such as a hospitalized patient at risk for antibiotic infection. We also believe that in the guts of forest insects, we will find naturally antibiotic resistant enterococcal species that may have been the source of some of the antibiotic resistances that found their way into human-associated enterococci.
Perspectives
Humans naturally focus their interest first on what is nearest around them. In microbiology, much of the focus has been on bacteria isolated from human infection. Covid reminded the world that there are important infection risks in nature, and as the human population grows and expands into new ecologies, we will be increasingly exposed to those risks. This is reminding us that life on the planet Earth is tightly interconnected. As we have mainly examined life in proximity to our daily activities, there is an important gap in understanding life elsewhere in nature that could profoundly affect us as we encounter it. The recent pandemic highlights the urgency in filling this gap in knowledge. Antibiotic resistance already is one of the known leading threats to human health, and as antibiotics have existed in nature for hundreds of millions of years compared to the hundred years of human association, it is critical for us to understand the impact of antibiotics and antibiotic resistance on natural ecologies to be able to better predict how they will impact human health. In the process we may well discover new information that will help curb the spread of antibiotic resistance.
Michael Gilmore
Massachusetts Eye and Ear Infirmary
Read the Original
This page is a summary of: Global diversity of enterococci and description of 18 previously unknown species, Proceedings of the National Academy of Sciences, February 2024, Proceedings of the National Academy of Sciences,
DOI: 10.1073/pnas.2310852121.
You can read the full text:
Resources
Contributors
The following have contributed to this page