What is it about?
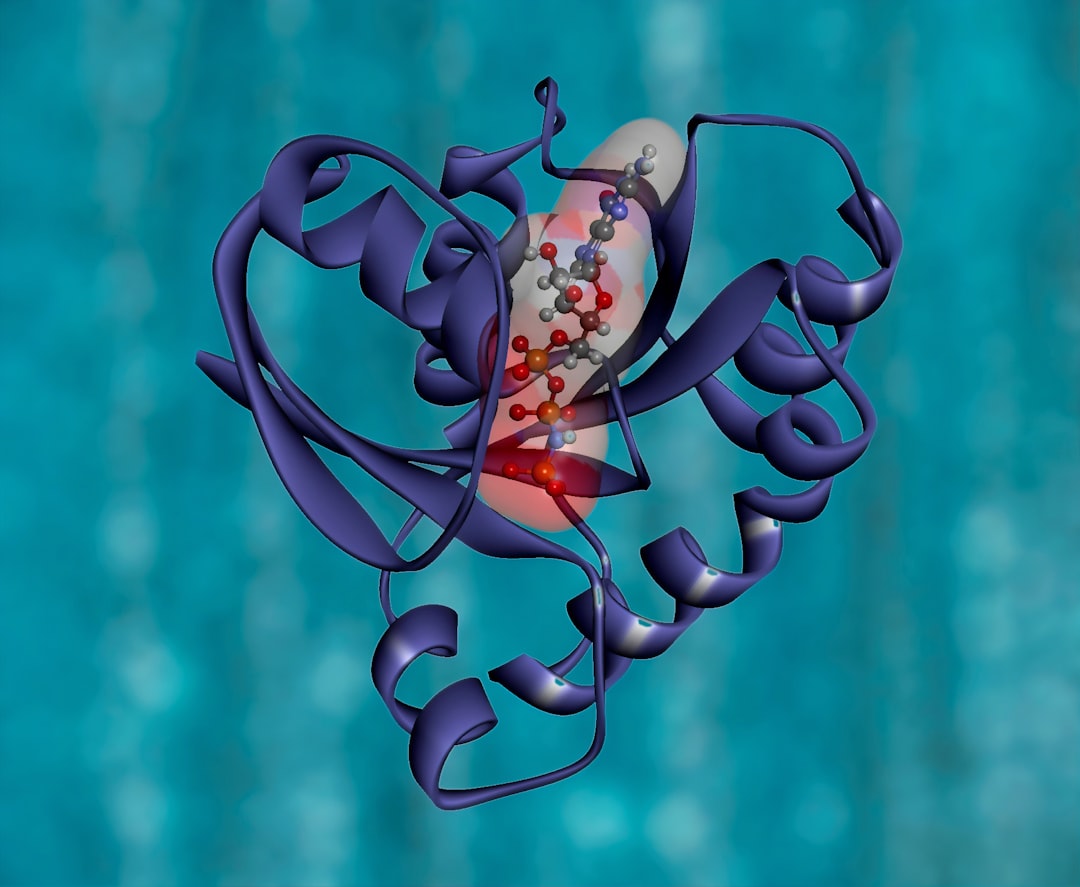
Transglycosylases are an emerging tool for oligosaccharide synthesis. Transglycosylation relies on subtle molecular factors that are still ill-described. We have obtained for the first time a structure of a GH5 enzyme in complex with transglycosylation products. This study has showed how the sugar acceptor length influences transglycosylation.
Featured Image
Why is it important?
Thanks to structural data, we show that transglycosylation depends on the sugar acceptor and donor length. In RBcel1, transglycosylation is more effective with cellotriose (three glucose units) as acceptor than with cellobiose (two glucose units). At the structural level, cellotriose is 0.8 A closer to the sugar donor compared to cellobiose. The occupation of the acceptor subsites is critical for transglycosylation to occur.
Perspectives
I took pleasure writing this article with my co-authors. it was an exercise of style to present those wonderful structures. I hope that people will enjoy reading our article and find the soundness of data.
PhD Raphael Dutoit
LABIRIS
Read the Original
This page is a summary of: Highlighting the factors governing transglycosylation in the GH5_5 endo-1,4-β-glucanase RBcel1, Acta Crystallographica Section D Structural Biology, February 2022, International Union of Crystallography,
DOI: 10.1107/s2059798321013541.
You can read the full text:
Resources
Contributors
The following have contributed to this page