What is it about?
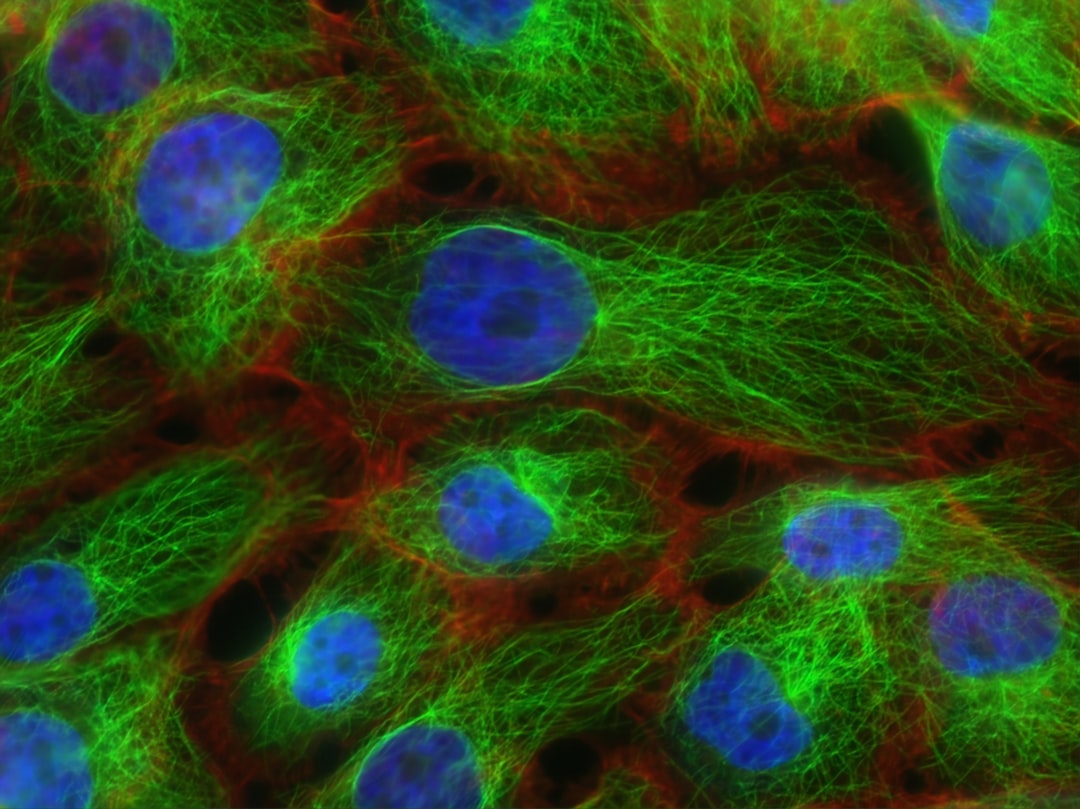
Background: Cytoskeleton anchoring of conformational mutant-like p53 is prominent in human senescent cells. The present research investigated the structural basis of vimentin cytoskeleton- anchoring of human p53. Method: GFP-fused wild type p53, mutant p53, those of the various truncated isoforms including p53β and p47, were expressed in the vimentin-expressing cells: mouse fibroblasts, COS-7 cells, young and senescent human fibroblasts, and HeLa cells (non-vimentin-expressing).
Featured Image
Why is it important?
Results: A cancer-specific mutant p53V143A-GFP and p53R249S-GFP expressed in mouse fibroblasts, exclusively anchored on the vimentin cytoskeleton. Thus, the cancer-specific mutant p53R249S and p53V143A adopt distinct mutant conformation and thereby the C-terminal region (amino acid 331-360) potently interacts with the vimentin cytoskeleton and HeLa cells' cytoskeleton. Wild type p53-GFP exclusively localized in the nuclei of growing young fibroblast, in contrast to the significant cytoplasmic retention in senescent human fibroblasts. The deletion of p53 at the N-terminus or at the C-terminus (ΔN40 or ΔC63) results in a significant nuclear import of the shorter isoforms, p53β and p47. Conclusion: Senescent fibroblasts promote p53 to adopt a hotspot mutant like-conformation which significantly overrides the nuclear import due to the potent cytoskeleton-anchoring. Interestingly, the shorter p53 isoforms can escape from the cytoskeleton-anchoring.
Perspectives
Human senescent fibroblasts significantly retained the cytoplasmic aggregates of wild type p53. Wild type p53 aggregation is similar in mechanism to the aggregation propensity of the highly destabilized mutant p53. Senescent fibroblasts modify or destabilize p53 so that it adopts a mutant-like conformation preferring the aggregation. Accordingly, the importance of Pin1 catalyzing protein folding and conformational changes of p53 is discussed. Folding of wild type p53 is promoted by an interaction with the chaperonin CCT. Finally, It is an interesting hypothesis that the depletion of CCT or Pin1 in the senescent cells may prime conformational mutant-like p53the and lead to the aggregation of destabilized p53.
Dr Koji Nishio
Nagoya Daigaku
Read the Original
This page is a summary of: Cytoskeleton-Anchoring of Conformational Mutant-Like p53, but Not Shorter Isoforms p53β and p47 (ΔN40p53) in Senescent Human Fibroblasts, Current Aging Science, October 2017, Bentham Science Publishers,
DOI: 10.2174/1874609810666170523153639.
You can read the full text:
Contributors
The following have contributed to this page