What is it about?
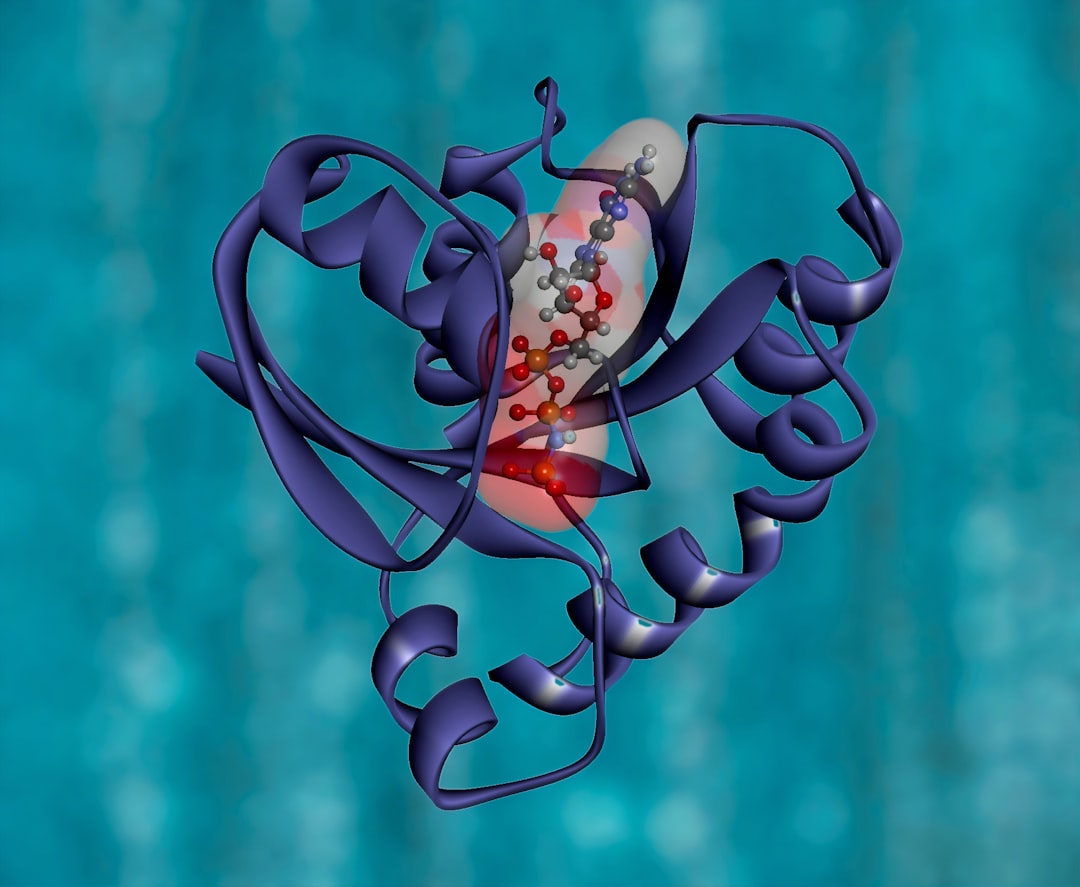
There are no clinically useful inhibitors of metallo-β-lactamases (MBLs), which are a growing problem because they hydrolyse almost all β-lactam antibacterials. Inhibition by most reported MBL inhibitors involves zinc ion chelation. A structure-based virtual screening approach combined with NMR filtering led to the identification of inhibitors of the clinically relevant Verona Integron-encoded MBL (VIM)-2. Crystallographic analyses reveal a new mode of MBL inhibition involving binding adjacent to the active site zinc ions, but which does not involve metal chelation.
Featured Image
Why is it important?
The results will aid efforts to develop new types of clinically useful inhibitors targeting MBLs/MBL-fold metallo-enzymes involved in antibacterial and anticancer drug resistance.
Perspectives
The findings show the inhibition of metallo-β-lactamases by non-metal chelating inhibitors, an unprecedented mechanism of inhibition.
Martine I Abboud
University of Oxford
Read the Original
This page is a summary of: NMR-filtered virtual screening leads to non-metal chelating metallo-β-lactamase inhibitors, Chemical Science, January 2017, Royal Society of Chemistry,
DOI: 10.1039/c6sc04524c.
You can read the full text:
Contributors
The following have contributed to this page