What is it about?
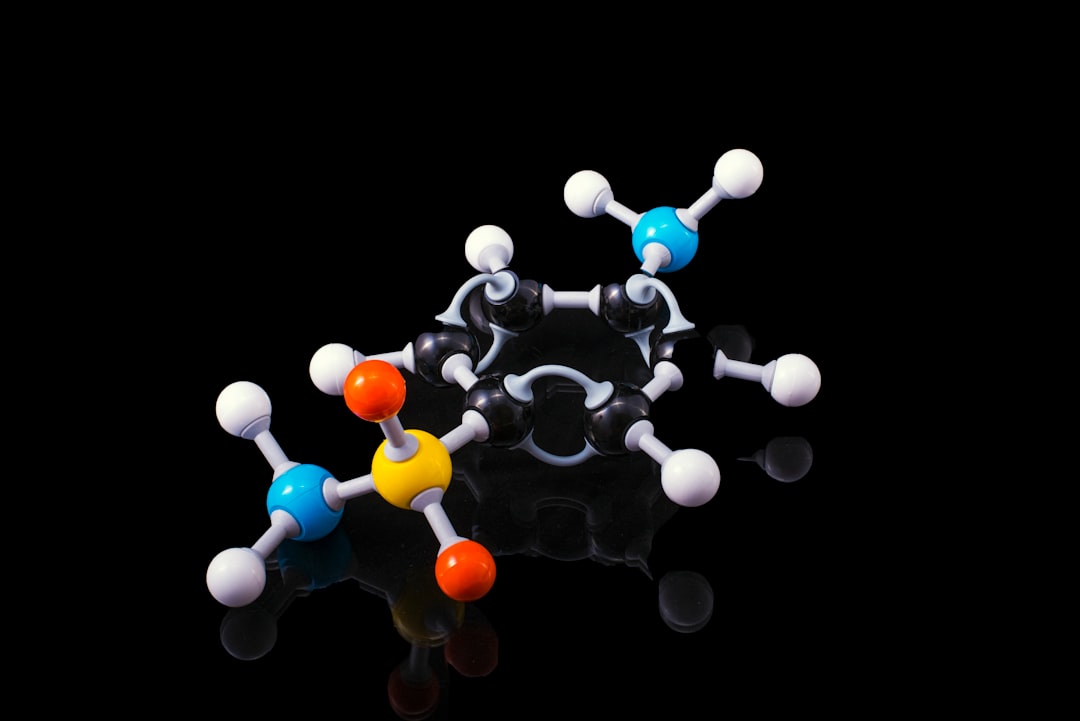
The systematic structural analysis of cyclic organoaluminum compounds, 1-ethyl-3(R)-substituted alumolanes (R = n-Bu, n-Hex, n-Oct, i-Bu, Ph, Bn, SiEt3, cyclohexenyl) and 1-ethyl-2-phenylalumolane in polar solvents (Et2O, THF, pyridine), has been carried out by the means of NMR 1H, 13C, 27Al spectroscopy and quantum chemical methods (DFT, MP2). The NMR spectroscopic criteria for identification of alumolanes were established. Predominant conformation of the metallacycle as twist form with pseudo-equatorial location of a substituent was defined using experimental and theoretical approaches. The direct heteronuclear coupling constants J (1H-13C) in alumolanes were found. Relative stability of the alumolane solvates has been determined on the basis of calculated thermodynamic parameters of complexation reaction and from the dependence of the 1H, 13C, and 27Al NMR chemical shifts of metallacycle atoms vs the solvent nature.
Featured Image
Why is it important?
Five-membered saturated metallacarbocycles represent a large family of organometallic compounds, which are frequently postulated as reactive intermediates in catalysis. We found that there is a lack of reliable 1H, 13C, or 27Al NMR structural information on aluminacycles in the literature. For the first time, conformations of 2-substituted and 3-substituted alumolanes, as well as the dynamics of their behavior in polar media were defined.
Perspectives
The work is of fundamental significance, and it contributes into the understanding of the structure and dynamics not only cyclic organometallic substances but heterocarbocycles (borolanes, phospholanes), since found effects and regularities may be common to both classes of compounds.
Dr Tatyana Victorovna Tyumkina
Read the Original
This page is a summary of: Structure and conformations of 2-substituted and 3-substituted alumolanes in polar solvents: a direct NMR observation, Magnetic Resonance in Chemistry, August 2015, Wiley,
DOI: 10.1002/mrc.4311.
You can read the full text:
Contributors
The following have contributed to this page